
Graduate Student in Physics
am studying water in confined systems using
computer simulations. My first project is a constant pressure and
temperature molecular dynamics study of water between two plates
acted upon by an external electric field. This system is immersed
in a larger "bath" with which it is in chemical and thermal
equilibrium.
These simulations show a very different behavior
of water compared to constant volume simulations. Our results
imply that for fluids with large molecular dipole moments like
water, current ideas of electrostriction need
revision.
The second project is a Monte Carlo study of single-file water chains
inside a carbon nanotube. We have studied the structure and
energetics of the confined water at different electric fields and
temperatures.
By evaluating the grand-canonical partition
function we show that external electric fields favor the filling
of previously empty nanotubes from the bulk phase. A simple
two-state statistical mechanics model provides an excellent
approximation for the electric field dependence of the
polarization of the water chain and its effect on the filling
equilibrium. We also find that the filled state of the tube is
favored by increasing temperature near ambient conditions. This
implies, counter-intuitively, that the entropy of the confined
water is greater than that of bulk water.
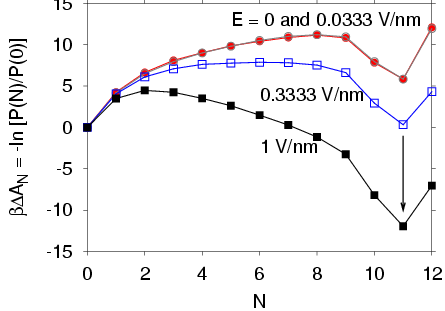
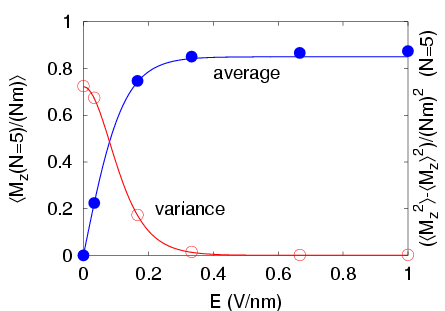
Electric field dependence of the total dipole moment of the water chain.
Comparison of simulations with the two-state model.
